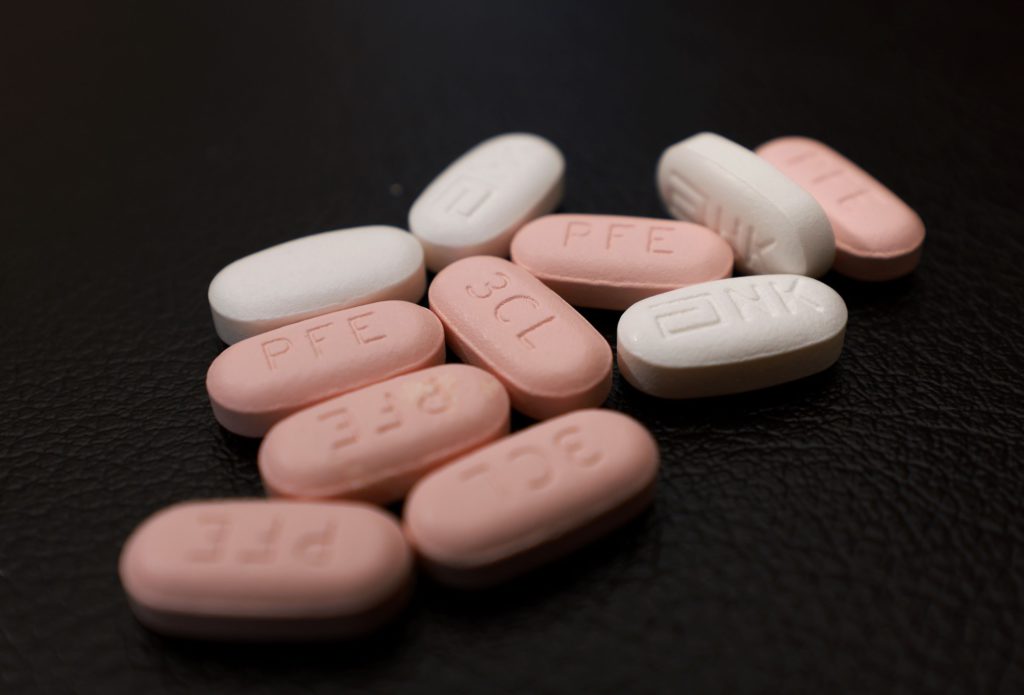
(Bloomberg) — Pfizer Inc. and BioNTech SE submitted early-stage data to U.S. regulators showing that a third dose of their Covid-19 vaccine led to higher levels of protective antibodies when given eight to nine months after the initial regimen.
The companies expect results from a larger final-stage trial evaluating the effects of the third booster dose shortly, according to a statement Monday, which will be submitted to the U.S. Food and Drug Administration, the European Medicines Agency and other regulatory authorities.
Last month, Pfizer said it would approach U.S. regulators for emergency-use authorization of a third booster dose of its vaccine, based on early data showing that it can sharply increase immune protection against the coronavirus and variants, including delta.
Now, the companies are pursuing a different path toward booster clearance, seeking formal approval rather than emergency authorization. Pfizer and BioNTech said they plan to seek licensure of the third dose through a supplemental Biologics License Application in people 16 years old and up, pending approval of their primary application submitted in May.
Shares of New York-based Pfizer were little changed at 1:23 p.m. in New York, while Mainz, Germany-based BioNTech’s American depository receipts were down 12%, continuing a decline that began before the statement.
Booster Debate
Debate is accelerating in the U.S. and Europe over whether booster shots will be needed, and if so, when and in which subgroups of patients. U.S. cases are rising sharply thanks both to the highly transmissible delta variant and large numbers of unvaccinated people. Even as some vaccine breakthrough cases occur, the vast majority of U.S. hospitalizations and deaths have occurred in people who didn’t get the vaccine.
Read More: Fauci Says U.S. Can Move Quickly to Offer Third Vaccine Shots
Pfizer has cited data suggesting the efficacy of its vaccine against mild cases may start to fade after around six months, even though protection against severe cases remains strong. Meanwhile, Moderna Inc. has said trial results show that its vaccine held up well over six months. But executives from both companies have argued booster shots will eventually be needed.
Last week, U.S. regulators authorized a third dose for immunocompromised people, such as organ transplant patients and people receiving cancer chemotherapy. The case for a third dose in this group is strong because unlike healthy people, many of the immunocompromised never get a good response to the first two vaccine doses.
(Updates shares in fifth paragraph)
More stories like this are available on bloomberg.com
©2021 Bloomberg L.P.